
This year marks the 27th anniversary of Autobio Diagnostics Co., Ltd (hereafter "Autobio"). Through 27 years of relentless endeavor, Autobio has been continuously breaking new ground and moving forward with courage in the field of in-vitro diagnostics (IVD) with tenacity and a pursuit of excellence. Today, we formally launch Autobio's "No.1" Series of articles, systematically charting Autobio's landmark achievements in core-technology research and development and high-end product innovation. Via vivid RD narratives and practical timelines, we retrace the company's evolution in multiple technology domains from "following" and "running alongside" to "leading the way", thereby showcasing the innovation capabilities of a Chinese IVD enterprise. The inaugural article of the "No.1" Series will focus on Autobio's strategic product —— the TLA System in Medical Laboratory.
In 2017, Autobio succeeded in launching China's first independently-branded TLA System Autolas A-1 Series. In 2022, the wholly self-developed and self-manufactured Autolas X-1 Series was introduced, with a "three-step" strategy that took TLA system in medical laboratory from zero to a fully local RD and manufacturing capability.
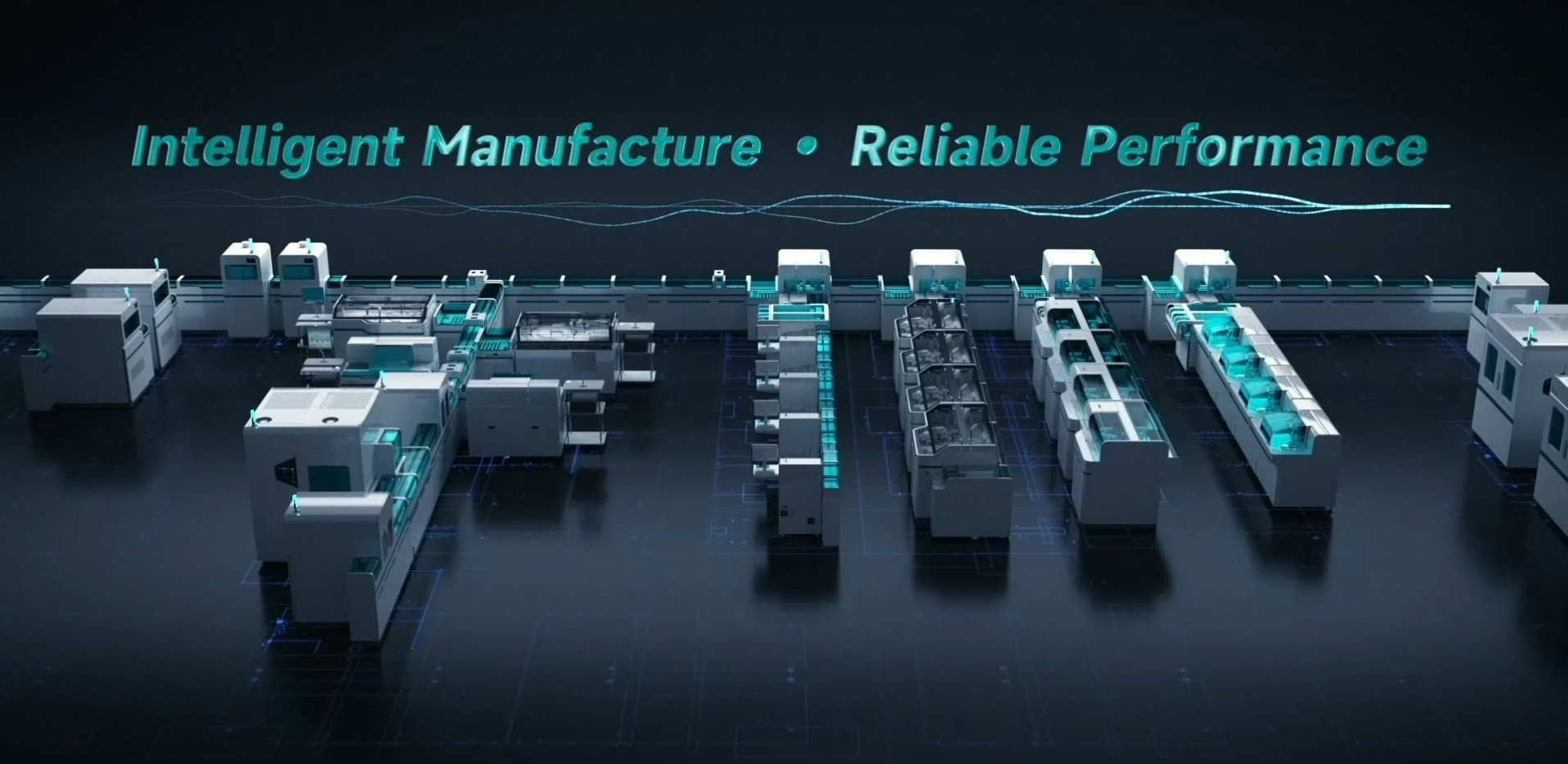
The TLA system in medical laboratory is a comprehensive system: they encompass sample handling, transport, and storage; immunoassay, biochemistry, coagulation analyzers and reagents; and they require a suite of analysis, quality-control and integrated-control software to drive coordinated operation. As the key enabler of intelligent medical laboratories, they are aptly termed the "jewel in the crown" of the IVD industry.
Laboratory automation began in the 1950s; by the 1980s, Japan, responding to a shortage of clinical-laboratory technicians, built the world's first TLA line, which subsequently entered the European and American markets and gradually became prevalent in developed countries globally. In 2001, the First Affiliated Hospital, Zhejiang University School of Medicine introduced the Hitachi PAM+7600 system, thereby establishing China's first biochemical automation line. As healthcare standards in China continue to rise, growing medical demands are being unleashed, with patient numbers and sample volumes increasing year by year. TLA system are now rapidly expanding domestically, thanks to their automation and intelligence.
Initially, TLA system were entirely dominated by international companies, with no domestic firm capable of developing and producing such solutions under its own brand. Recognizing this gap, Autobio began laying the groundwork for its own TLA system as early as 2011.
First, Autobio partnered with Canon Medical to acquire high-quality biochemical analyzers; and acquired Bio-Top to obtain high-quality, comprehensive biochemical reagents, thereby quickly building a biochemical-testing product line.
Next, Autobio integrated sample transfer modules with detection modules. Through intense negotiations with a Japanese partner, it introduced advanced magnetic levitation tracks and pre- and post-processing systems via OEM, combined with its own detection modules. In 2017, Autobio became the first in China to launch the magnetic levitation laboratory automation lines, Autolas A-1 Series, breaking the monopoly of international giants in this field.
Autobio then began development of a localized TLA system. After visiting nearly one thousand medical laboratories and documenting more than four thousand customer requirements and suggestions, it unveiled the self-developed, self-manufactured TLA system Autolas X-1 Series in 2022.
The product features an innovative three-dimensional, recyclable triple-track design, effectively reducing sample congestion on the line. Each single-module centrifuge can process up to 450 tubes per hour and supports multi-module expansion, meeting diverse laboratory centrifugation needs. Equipped with the highest-capacity online refrigerator per unit area, a single module can store over 15,000 samples, covering most labs' week-long storage requirements. In addition, the Autolas X-1 Series won the 2023 IF Design Award in Germany and the 25th China Patent Award - Excellence Award for Outstanding Industrial Design——recognition not only for its innovative design but also for Autobio's commitment to independent RD and advancing the industry.
In 2025, to resolve the spatial and efficiency constraints of outpatient departments and smaller laboratories, Autobio introduced the Autolas B-1 Star intelligent TLA system: this product occupies minimal floor-space, is fully featured, and high-efficiency; it integrates front- and rear-sample processing functions, connects to biochemical, immunoassay and coagulation analyzers, and is applicable in diverse laboratory application scenarios.
Autobio's three-step strategy enabled it to produce TLA system from scratch and evolve from a domestic brand to fully independent RD and manufacturing. This accomplishment broke the longstanding grip of international giants, revitalized China's IVD industry and set the pace for domestic development.

Both the range of tests offered and the underlying expertise they embody are critical considerations when selecting a TLA system. Autobio's TLA system now support more than 300 testing assays spanning biochemistry, immunoassay, coagulation, and beyond, ranking it among the companies with the broadest test offerings in China.
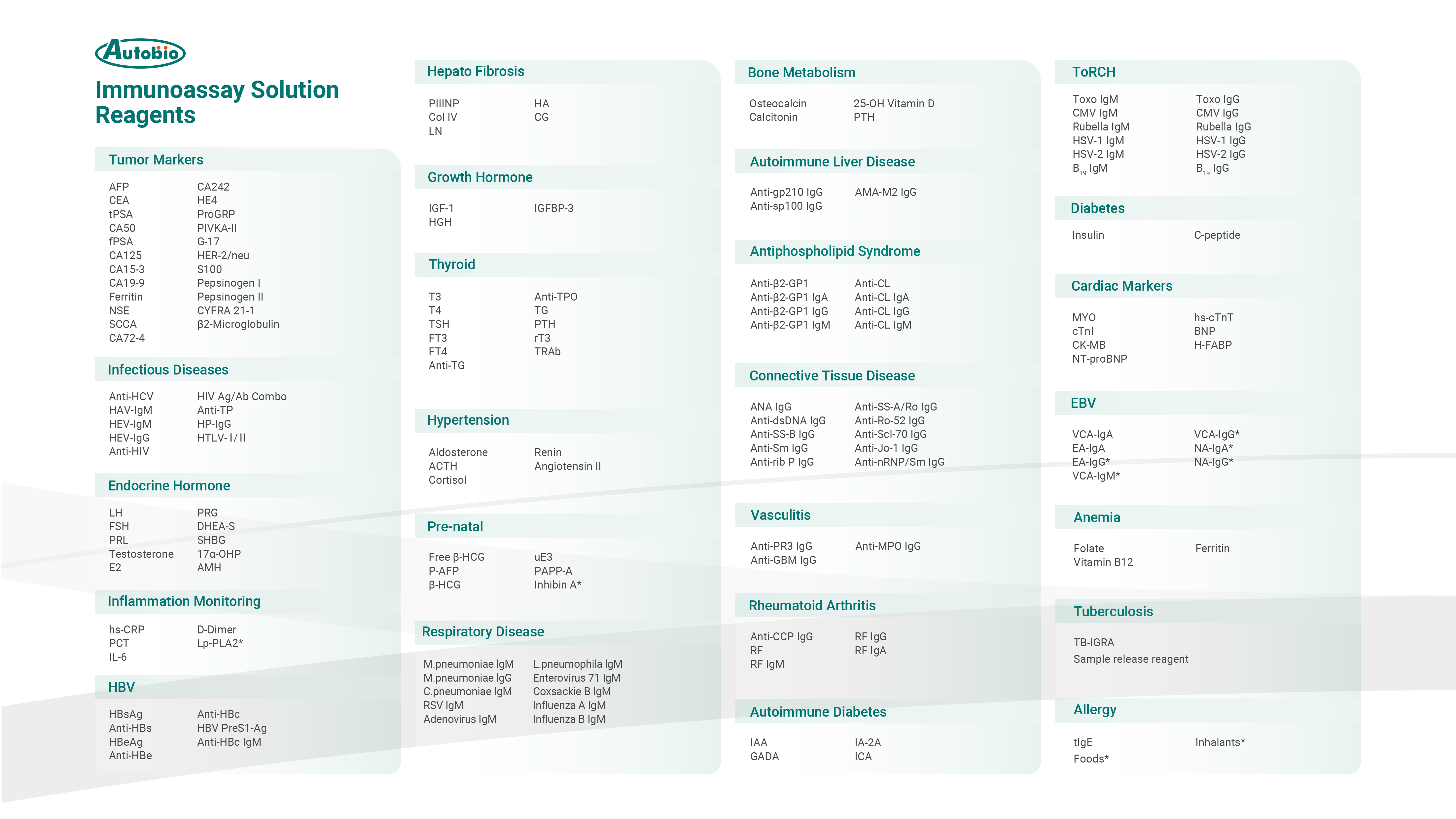
In the immunoassay testing field, Autobio possesses profound technical foundation and a comprehensive menu: more than 170 items, and a core raw-material self-supply rate that reaches an internationally leading level. Going forward, Autobio will continue its "strategic outpost" approach to RD, and consistently roll out new immunoassay tests.

In the field of biochemical testing, 128 assays are available, covering routine biochemical analyses; in coagulation testing field, 7 assays are offered, encompassing fundamental indicators of coagulation function. Additionally, Autobio is undertaking the National Key RD Program project titled "Development of a High-Performance TLA System". In the future, it will integrate 7 testing technology platforms, including immunoassay, biochemistry, molecular diagnostics, coagulation, hematology, mass spectrometry, and flow cytometry. The immunoassay and biochemical testing portfolio is expected to exceed 500 assays, aiming to establish a TLA system with the "largest number of platforms" and the "most comprehensive array of assays".
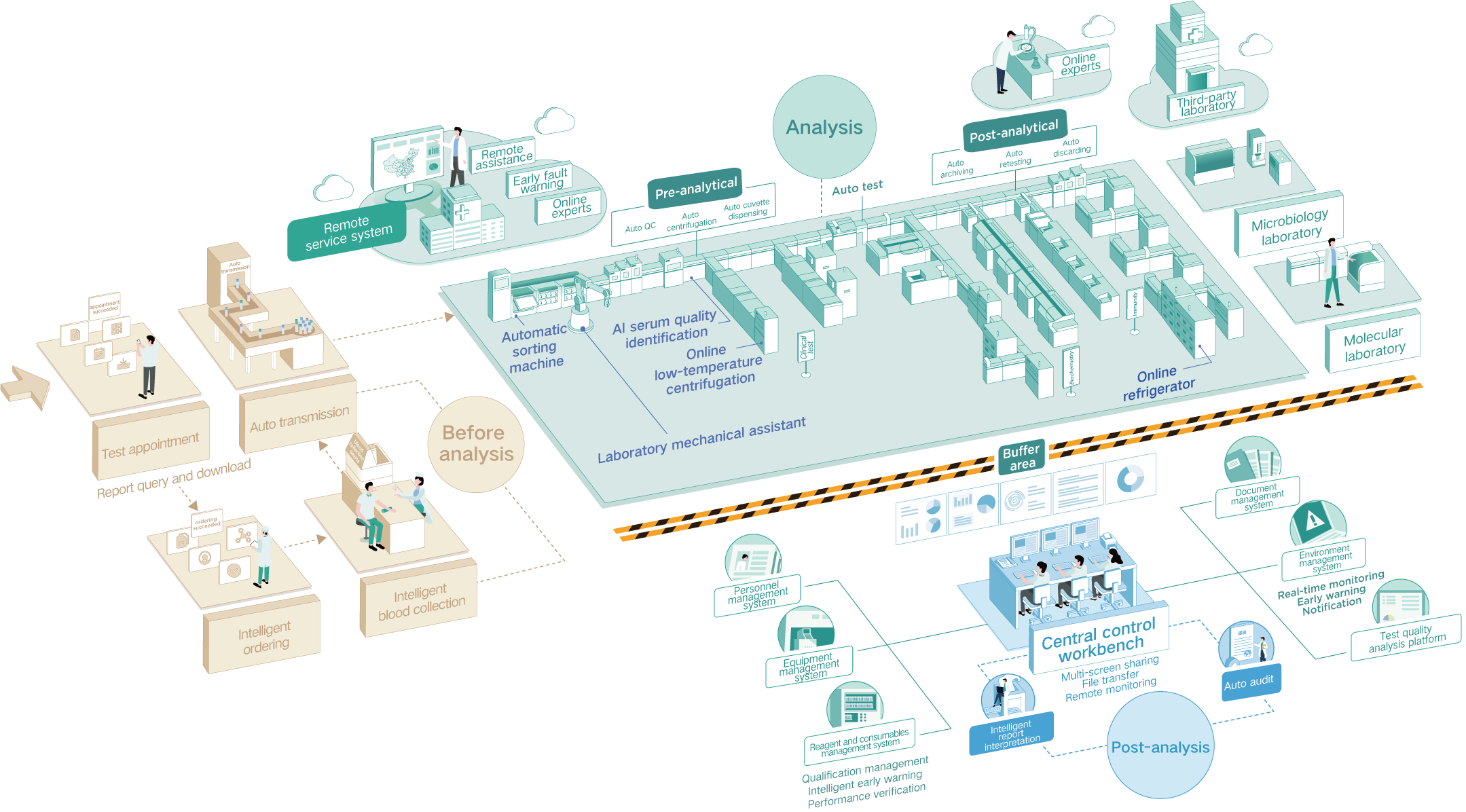
As technology accelerates, artificial intelligence, big data, and cloud computing are increasingly shaping all sectors, and the IVD industry is no exception. Autobio is investing in intelligent lab solutions to create more competitive offerings.
Autobio independently developed a middleware control system for its TLA system, enabling functions such as sample load balancing, critical value management, automatic sample retesting, and result verification. This system provides precise end-to-end control over sample processing, significantly optimizing workflow and enhancing operational efficiency. Pioneering within the industry, Autobio also introduced AI-based recognition technology to automatically assess serum quality. Trained on over 60,000 clinical serum images, this system effectively reduces errors inherent in manual visual inspection and addresses the challenge of inconsistent evaluation standards, thereby ensuring the reliability and precision of test results.
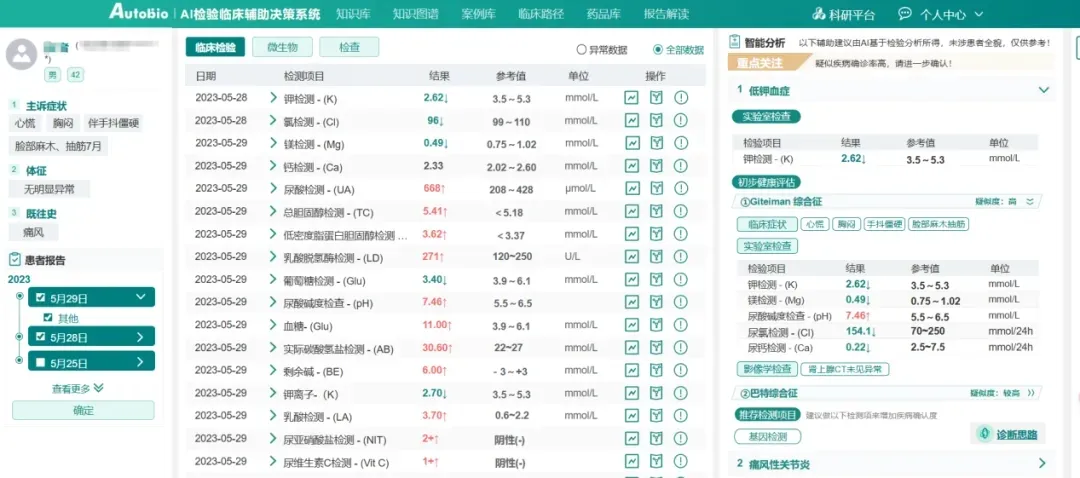
Autobio's newly launched AI Clinical Laboratory Decision Support System integrates a comprehensive laboratory knowledge base and clinical pathway query functions. It supports over 5,000 items and more than 3,000 disease interpretations, which further enhances healthcare quality and efficiency. Looking ahead, Autobio plans to build a knowledge-based large model tailored for laboratory diagnostics to provide smarter clinical decision support.
Beyond providing TLA solutions, Autobio brings lean management practices into labs. By virtue of 6S implementation, process optimization consulting, and lean project improvement services, it delivers full-scale professional lean services for medical laboratories.
From the early challenges, to breaking international monopolies, to achieving a significant leap in domestic RD and manufacturing, and now expanding into intelligent lab solutions, Autobio has continuously pushed boundaries with innovation. Backed by deep technical expertise and sustained RD investment, it has earned the IVD's "Jewel in the Crown". Looking ahead, Autobio will continue to embrace innovation, deepen its presence in IVD, and contribute further to the smart development of China's medical laboratories and the improvement of healthcare services, thus creating new milestones in its journey.
