
Autobio Diagnostics Co., Ltd (Hereinafter called Autobio) was founded in 1998, headquartered in National Eco & Tech Zone, Zhengzhou.
Autobio specializes in R & D, production, marketing, and service of clinical diagnostic products, especially covering immunoassay, microbiology, and biochemistry fields. Meanwhile, Autobio is also actively planning developments in the field of molecular diagnostics and provides a comprehensive solution for medical laboratories. Autobio was listed on Shanghai Stock Exchange on September 1st, 2016, and is the first IVD manufacturer listed on the main board in Shanghai, China. In 2024, Autobio's revenue achieved RMB 4471 million.

R & D experience
2024 operating income
HQ/IDC/R&D Center
EMPLOYEES

The annual average R&D investment exceeds 15% in the last year; Autobio focuses on team building, with over one-third of R&D talents among all employees; Compared with the international advanced experience, Autobio applies strict research and development process management; Focusing on the research and development of core raw materials, Autobio has created a diagnostic antibody library for tens of thousands of antigen epitopes, with over 73% self-supply for antigen and antibody, which ensures the stability and safety of supply. Autobio has become one of the leading companies with the most registered products and the most comprehensive product catalog.
registration certificates
CE certifications
Patents
Customers come first
value creation for customers
Autobio takes "customers come first, value creation for customers" as its service concept and insists on improving service standards and service ability. Autobio has over 800 professional service engineers to provide timely and thoughtful technical services to customers across the country. While providing customers with products & technical services, Autobio also provides medical laboratories with Internal Quality Control and integrated service.
Autobio is always taking product quality and sophisticated manufacturing as development priorities. In terms of reagent production, an automatic production line is introduced to improve the level of product automation and workflow, and improve the level of refined management; In terms of instrument manufacturing, Autobio will continue to strengthen the refined management and advocate the spirit of artisans to pursue excellence and continuous improvement; In terms of quality management, Autobio has passed the GMP, ISO9001 and ISO13485 certification, and strict quality management leads to continuously quality improvement.
While focusing on independent innovation and sophisticated manufacturing, Autobio also focuses on global resource integration. Through the integration of research and development innovation resources and even advanced technologies in the industry, Autobio can provide more abundant products for medical laboratories.

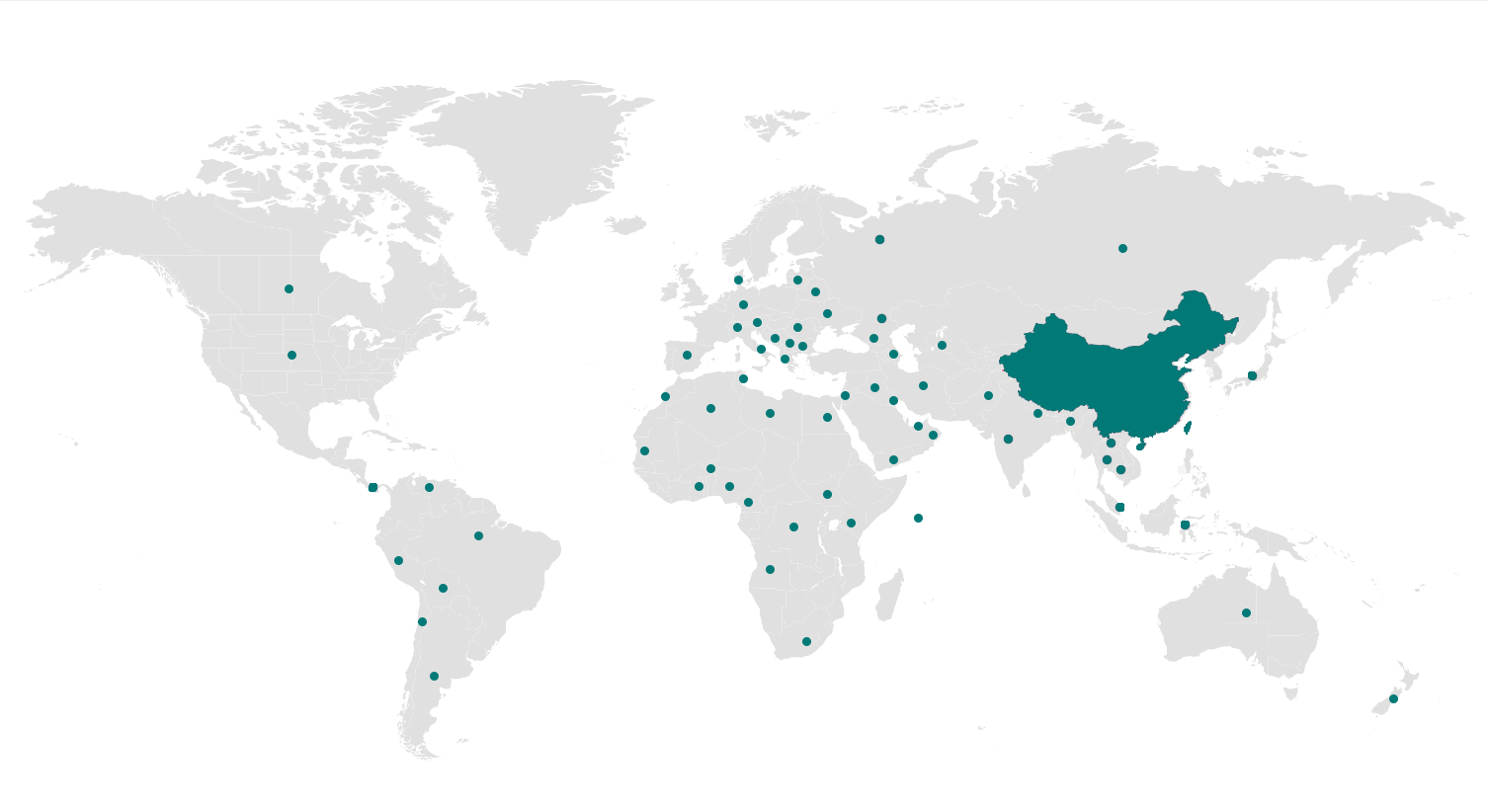







Autobio's marketing network covers the whole country, and our products have entered more than ten thousand medical institutions. In overseas markets, Autobio's products have performed enormous potential, exporting to over 100 countries, such as Europe, Asia, the Middle East, South America, Africa, etc.
Under constructed Autobio industrial park covers an area of more than 250 acres, with a construction area of more than 500,000 square meters(more than 100,000 square meters have been put into use). Upon completion, it will have over US$ 2.2 billion production capacity and will become one of the largest IVD bases in China.
acres
square meters
billion
Address: NO.87 Jingbei Yi Rd, National Eco&Tech Zone, Zhengzhou, China
Email: info@autobio-diagnostics.com Tel: +86-371-6200-7036
Autobio Copyright Reserved for ICP 18006568. All Rights Reserved.

