

Pancreatic ductal adenocarcinoma is one of the most difficult malignancies to diagnose and treat. The most widely used and best validated marker for pancreatic cancer is CA 19-9[1].CA 50 is a new tumor marker based on a monoclonal antibody (MAb) against a human colorectal carcinoma cell line[2]. The detection of serum tumor markers (CA19-9, CEA, CA125 and CA242) is conducive to the early diagnosis of pancreatic cancer and joint detection of tumor markers helps improve the diagnostic efficiency[3].
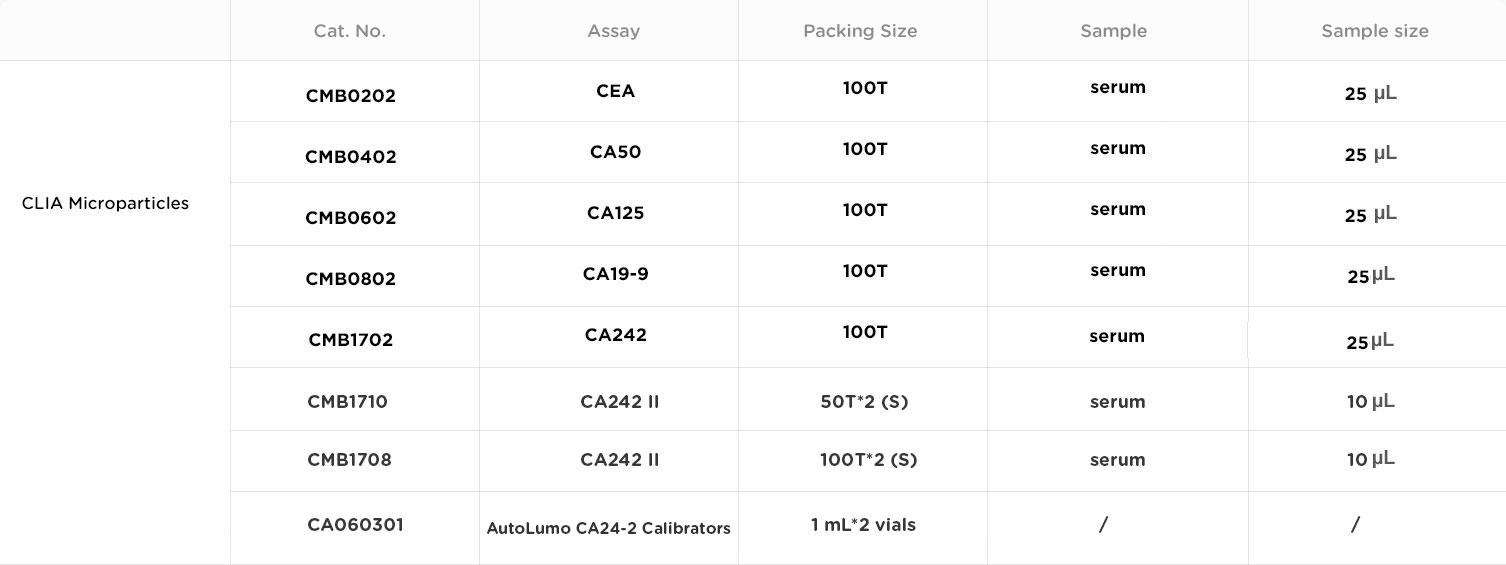
*Autobio is devoted to enhancing the quality of our products and services. Please refer to our product manuals for detailed specifications and parameters.
Although discovered 30 years ago, CA 19-9 remains the gold standard serum marker for patients with pancreatic cancer.[1] Follow-up using CA 19-9 and CA 50 is a simple and sensitive way of monitoring the postoperative course of patients with pancreatic cancer, and may give a lead time of several months for a recurrence compared to conventional methods.
CA242 and CA19-9 have better performance in the diagnosis of pancreatic cancer than CEA. Furthermore, parallel combination pattern of CA19-9+CA242 could be considered of better diagnostic value for pancreatic cancer patients[4].
CA50 is a glycolipid antigen that plays an important role in cell growth and differentiation. Subgroup analyses indicated that CA50 was the only tumor biomarker that was significantly correlated with long-term survival in CEA-normal CRC patients[2].
Increased levels of the CA 19-9 and CA 125 markers in patients with pancreatic pathological abnormalities usually indicates a malignant nature of the lesion[5].
Although carbohydrate antigen 19-9 (CA19-9) is the most important serum biomarker in pancreatic cancer, the diagnostic and prognostic value of CEA is gradually being recognized.Furthermore, high levels of serum CEA are significantly related to poor prognosis of patients with pancreatic cancer. Thus, the measurement of serum CEA, as a vital supplementary to CA19-9, is inexpensive, convenient, and necessary for monitoring this disease[6].
[1]M.J. Duffy, C. Sturgeon, R. Lamerz, A. Nicolini, O. Topolcan, V. Heinemann.Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Annals of Oncology. VOLUME 21, ISSUE 3, P441-447, MARCH 01, 2010.
[2]Caj Haglund,Pentti Kuusela,Hannu Jalanko,Peter J. Roberts.Serum ca 50 as a tumor marker in pancreatic cancer: A comparison with CA 19-9.IJC International Journal of Cancer39(4):477-481. doi.org/10.1002/ijc.2910390412.
[3]Yu-Lei Gu 1, Chao Lan, Hui Pei, Shuang-Ning Yang, Yan-Fen Liu, Li-Li Xiao. Applicative Value of Serum CA19-9, CEA, CA125 and CA242 in Diagnosis and Prognosis for Patients with Pancreatic Cancer Treated by Concurrent Chemoradiotherapy. Asian Pac J Cancer Prev. 2015;16(15):6569-73. doi: 10.7314/apjcp.2015.16.15.6569.
[4]Yimin Zhang, Jun Yang, Hongjuan Li, Yihua Wu, Honghe Zhang, Wenhu Chen. Tumor markers CA19-9, CA242 and CEA in the diagnosis of pancreatic cancer: a meta-analysis. Int J Clin Exp Med 2015;8(7):11683-11691.
[5]Grzegorz Ćwik, MD, PhD; Grzegorz Wallner, MD, PhD; Tomasz Skoczylas, MD, PhD; et al.Cancer Antigens 19-9 and 125 in the Differential Diagnosis of Pancreatic Mass Lesions. Arch Surg. 2006;141(10):968-973. doi:10.1001/archsurg.141.10.968.
[6]Qingcai Meng, Si Shi, Chen Liang, Dingkong Liang, Wenyan Xu,Shunrong Ji, Bo Zhang, Quanxing Ni, Jin Xu,and Xianjun Yu. Diagnostic and prognostic value of carcinoembryonic antigen in pancreatic cancer: a systematic review and meta-analysis. Onco Targets Ther. 2017; 10: 4591–4598.
Address: NO.87 Jingbei Yi Rd, National Eco&Tech Zone, Zhengzhou, China
Email: info@autobio-diagnostics.com Tel: +86-371-6200-7036
Autobio Copyright Reserved for ICP 18006568. All Rights Reserved.




