

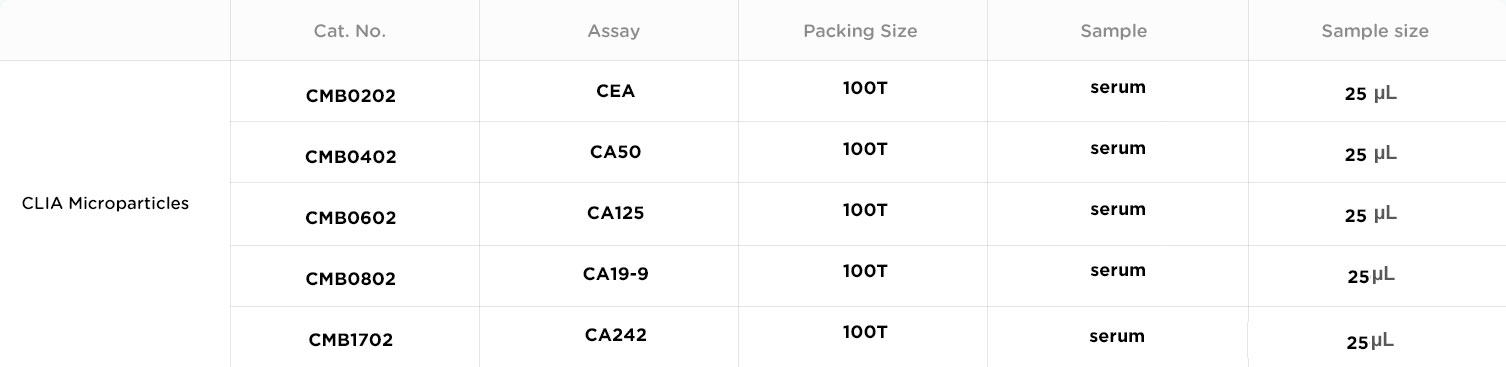
Colorectal cancer (CRC) is one of the most prevalent types of cancer, ranking as the third most common malignancy and the fourth leading cause of cancer deaths worldwide.[1]A wide range of serum tumor biomarkers, including CA19-9, CA242, CA72-4, CA50, and CA125, has been studied in association with colorectal cancer (CRC).[2]CEA and CA19-9 are the two most common tumor markers for colorectal cancer that are currently utilized clinically.[3]CA50 and CA724 could supplement CEA in monitoring recurrence and metastasis.CA125 represents a significant and independent prognostic factor in CRC patients, superior to CEA.[2]
*Autobio is devoted to enhancing the quality of our products and services. Please refer to our product manuals for detailed specifications and parameters.
Simultaneous use of the two markers is useful in evaluating the therapeutic effect and monitoring the recurrence of advanced colorectal cancer. [3]
ThereisapossibilityofusingCA242in monitoringdiseasestatusforpatientswithcolorectal cancer, being useful in differentiating benign vs. malignant disease as well as metastatic vs. non-metastatic cancer.[5]
CA50 is a glycolipid antigen that plays an important role in cell growth and differentiation. Subgroup analyses indicated that CA50 was the only tumor biomarker that was significantly correlated with long-term survival in CEA-normal CRC patients.[2]
CA125 concentration seems to be a better tumor marker than CEA concentration for predicting PD in CRC in both men and women. This finding suggests that CA125 concentration should be measured as part of the pretreatment evaluation.[6]
Combined serum markers can be used to not only diagnose colorectal cancer, but also appraise the tumor status for guiding treatment, evaluation of curative effect, and prognosis of patients.[4]
[1]Siegel R. L., Miller K. D. & Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians 66, 7–30, doi: 10.3322/caac.21332 (2016).
[2]Qingguo Li, Weixing Dai, Yaqi Li, YeXu, Xinxiang Li & SanjunCai. Nomograms for predicting the prognostic value of serological tumor biomarkers in colorectal cancer patients after radical resection.Scientific Reports,7:46345, DOI: 10.1038/srep46345.
[3]H Yamamoto 1, Y Miyake, S Noura, M Ogawa, M Yasui, M Ikenaga, M Sekimoto, M Monden. Tumor markers for colorectal cancer. Gan To Kagaku Ryoho. 2001 Sep;28(9):1299-305.
[4]Gao, Y., Wang, J., Zhou, Y. et al. Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and Ferritin as Diagnostic Markers and Factors of Clinical Parameters for Colorectal Cancer. Sci Rep 8, 2732 (2018).
[5]DANIELION,GEORGIANARADU,DANNICOLAEPĂDURARU, OCTAVIAN ANDRONIC, ALEXANDRA BOLOCAN. CA125 and CA242 markers' role in colorectal cancer diagnosis and management–a 30 year review. Romanian Biotechnological Letters 25(5):1977-1983. DOI:10.25083/rbl/25.5/1977.1983
[6]Huang, Chi-Jung PhD; Jiang, Jeng-Kai MD, PhD; Chang, Shih-Ching MD, PhD; Lin, Jen-Kou MD, PhD; Yang, Shung-Haur MD, PhD.Serum CA125 concentration as a predictor of peritoneal dissemination of colorectal cancer in men and women. Medicine e5177. doi: 10.1097/MD.0000000000005177
Address: NO.87 Jingbei Yi Rd, National Eco&Tech Zone, Zhengzhou, China
Email: info@autobio-diagnostics.com Tel: +86-371-6200-7036
Autobio Copyright Reserved for ICP 18006568. All Rights Reserved.




