
Recently, Autobio’s SARS-CoV-2 IgG CLIA Microparticle detection kit and SARS-CoV-2 IgM CLIA Microparticle detection kit obtained Registration Certification for Medical Device issued by National Medical Products Administration.
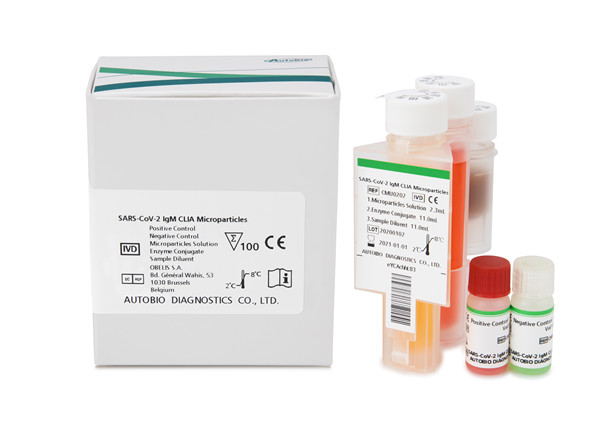
Both SARS-CoV-2 IgG CLIA Microparticle detection kit and SARS-CoV-2 IgM CLIA Microparticle detection kit can be applied on Autobio Fully Automatic Chemiluminescence Immunoassay Analyzer.


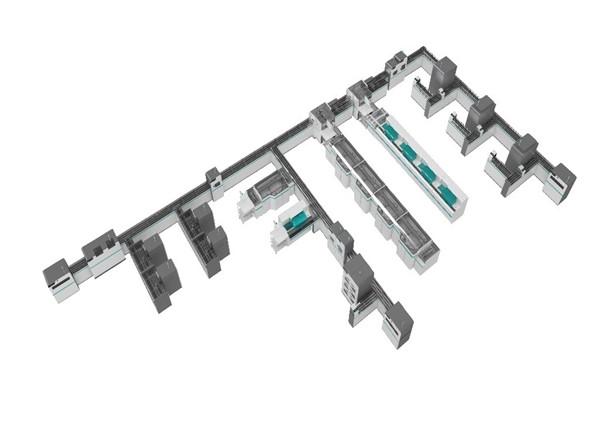
Autobio upholds the tenet of "committed to the penetration and improvement of medical laboratory technology,serving for human health" and strives to provide both cost-efficient and high-quality products to medical laboratories. Founded in 1998, Autobio has been focusing on the common development of both reagents and instruments. Product catalogue covers many IVD fields, including Immunoassay, Biochemistry, Microbiology, which can provide medical laboratories with comprehensive solutions and service.

Address: NO.87 Jingbei Yi Rd, National Eco&Tech Zone, Zhengzhou, China
Email: info@autobio-diagnostics.com Tel: +86-371-6200-7036
Autobio Copyright Reserved for ICP 18006568. All Rights Reserved.

