
In vitro diagnostics (IVD) medical devices play a crucial role in modern healthcare by providing essential diagnostic data that informs medical decisions. These companies are among the leaders in this sector, offering innovative technologies, stringent quality control, and a broad range of diagnostic tools. Here is a list of the top 10 IVD medical device companies in 2025, along with their profiles, locations, and main products.
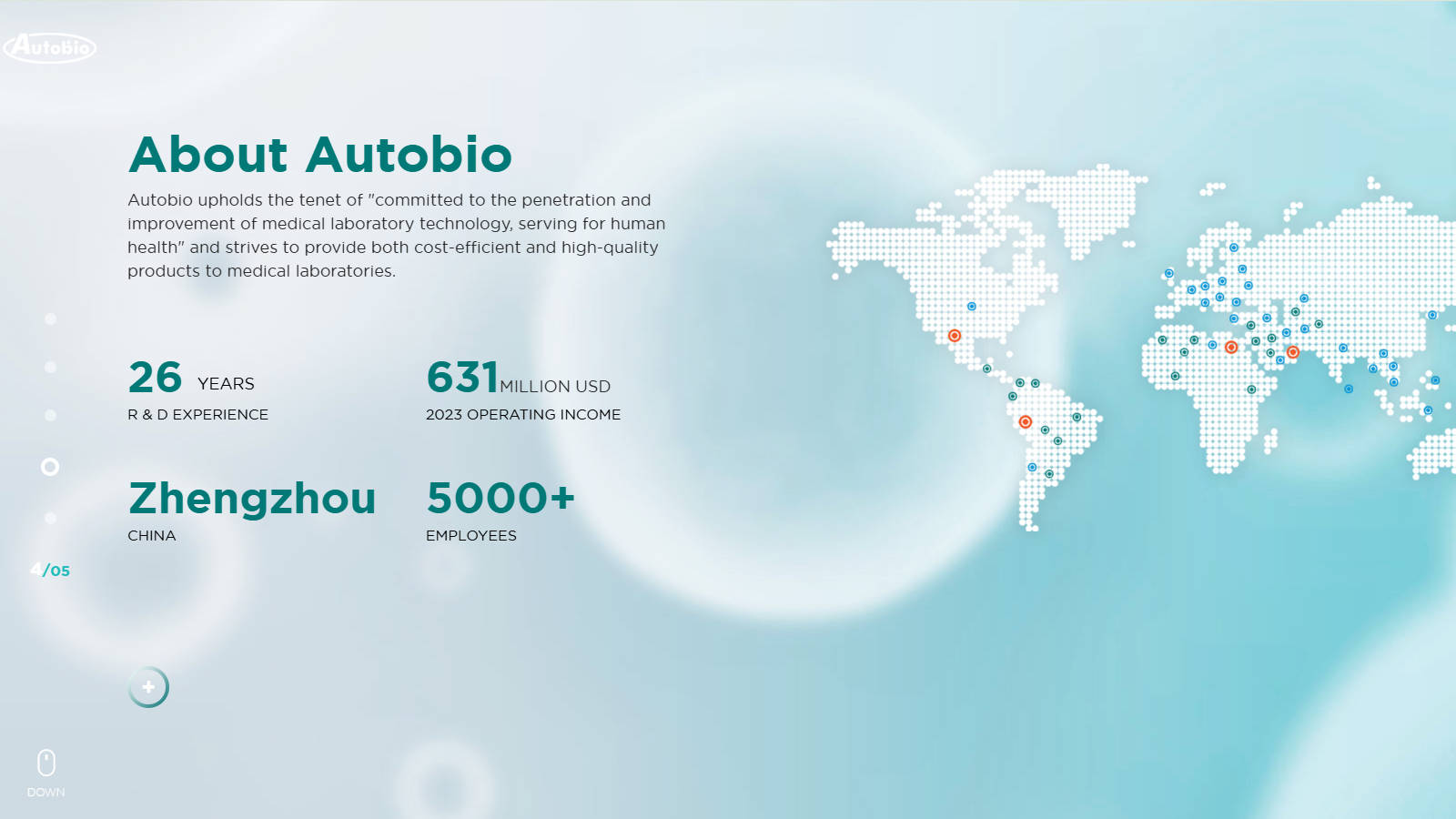
Company Profile: Autobio Diagnostics Co., Ltd (Hereinafter called Autobio) was founded in 1998, headquartered in the National Eco & Tech Zone, Zhengzhou. Autobio specializes in R & D, production, marketing, and service of clinical diagnostic products, especially covering immunoassay, microbiology, and biochemistry fields. Meanwhile, Autobio is also actively planning developments in the field of molecular diagnostics and provides a comprehensive solution for medical laboratories. Autobio was listed on the Shanghai Stock Exchange on September 1st, 2016, and is the first IVD manufacturer listed on the main board in Shanghai, China. In 2023, Autobio’s revenue achieved USD 613 million.
Location: 87 Jingbeiyi Rd, Guancheng Hui District, Zhengzhou, Henan, China, 450016
Main Products:
Company Profile: BIT Group is a leading provider of custom IVD medical device development and manufacturing services. With over 40 years of experience, BIT Group has earned a reputation for delivering high-quality solutions to some of the most significant global diagnostic companies. BIT designs, develops, manufactures, and services high-performance analytical IVD, medical, and life science devices for large global partners, well-established small-medium enterprises, and innovative startups.
Company Profile: Our customers rely on our quality products, prompt deliveries and expert advisors to do their jobs. Above all though, our customers rely on our knack for innovation. Our scientists and engineers tirelessly work to develop novel approaches to new and age-old challenges. We show our commitment to innovation through our financial investments as well. Innovation is visible when new products hit the shelves and when new services emerge. It’s also visible in our workspaces and new partnerships, driven in part by our M Lab™ Collaboration Centers.
Location: Ground Floor, Building 1, 885 Mountain Highway Bayswater 3153 Australia
Main Products:
Company Profile: We are a global genomics and human health leader innovating the future of precision health. We develop DNA sequencing and array-based life sciences technologies to enable boundless research discovery and personalized health. Our products help pioneer advances in oncology, genetic and infectious diseases, reproductive health, and beyond. Our technology empowers continued innovation towards positive and impactful people- and planet-healing solutions.
Location: 5200 Illumina Way (formerly 5200 Research Pl) San Diego, CA 92122 USA
Main Products:
Company Profile: MDR Regulator offers a full range of services enabling medical device manufacturers to place their products on European markets and to ensure continuity and compliance of their operations with applicable European laws. A company can use MDR Regulator's services at any stage of product launch - from design, through implementation, testing, documentation, certification, registration or notification, and post-market services (distribution, marketing, market surveillance and other activities).
Location: Al. Jerozolimskie 123A, 02-017 Warszawa
Main Products:
Company Profile: “IVD Research, Inc. and SafePath® Laboratories, LLC will develop, manufacture and sell its own line of in vitro diagnostics, which meet or exceed the performance of similar in vitro diagnostics in the marketplace. In addition, will provide contract development and/or manufacturing services which fulfill customer expectations and requirements.
Location: 5909 Sea Lion Place Suite D | Carlsbad, CA 92010 USA
Main Products:
Company Profile: We are quality makers – a team of experienced experts in healthcare and health tech quality. Our quality services for the healthcare, pharmaceutical and medical technology industries cover external quality assessments, regulatory consulting, clinical investigations and trials, audits and certifications, and training. Our expertise and knowledge benefit medical device and in vitro diagnostic manufacturers, pharmaceutical companies, healthcare units, and clinical laboratories.
Location: Kumpulantie 15 00520 Helsinki Finland
Main Products:
Company Profile: For over 30 years, we have been assessing the conformity of medical devices and quality systems enabling medical device manufacturers to place their products on the market worldwide. Our expertise in offering training courses tailored to your needs, the development of high-value technical and regulatory content, and the support provided throughout your certification cycle are all available assets for the success of your project. Choose GMED, its subsidiaries, and its parent company, LNE, and have reputable and recognized organizations in the field of certification and testing as your strategic partner.
Location: 1 Rue Gaston Boissier, 75015 Paris, France
Main Products:
Company Profile: An IVD medical device is defined in the IVDR as “any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body”. The definition then outlines the principle or sole purpose of these devices: under the IVDR, an IVD medical device must have a medical application or purpose.
Location: 1 Rue Gaston Boissier, 75015 Paris, France
Main Products:
Company Profile: In 1866, our founders had a bold vision to reduce the impact of technological risks and protect people, assets and the environment. More than 150 years on, sustainability and safety continue to be the backbone of our mission and services. Our aim is to inspire trust in technology, enabling progress by managing technology-related risks and facilitating change. Today, we are represented by more than 25,000 employees located across over 1,000 locations. Our community of experts is passionate about technology and is inspired by the possibilities of your business. United by the belief that technology should better people’s lives, we work alongside our customers to anticipate and capitalize on technological developments.
Location: 401 Edgewater Place Suite 500 Wakefield, MA 01880
Main Products:
As the landscape of IVD (In Vitro Diagnostic) medical device companies continues to evolve in 2025, our company stands at the forefront, committed to innovation, quality, and customer-centric solutions. With a track record of excellence in manufacturing cutting-edge diagnostic technologies, we offer unmatched precision and reliability in the IVD medical device industry. As highlighted in this guide to the top 10 IVD medical device companies, our unique approach to product development, adherence to rigorous standards, and continuous improvement set us apart from others. Whether you are looking for state-of-the-art diagnostic tools or seeking a trusted partner for your healthcare solutions, we pride ourselves on delivering the highest quality IVD devices that cater to global healthcare needs.