

The primary objective of susceptibility testing in clinic is to predict the treatment effect of an infected individual with chosen antimicrobial agents. Results must be derived in a timely manner with a high degree of accuracy and reproducibility. Antimicrobial susceptibility testing (AST) is usually carried out to determine which antimicrobial agent will be most successful in treating a bacterial infection in vivo. Clinical laboratories can choose to determine the susceptibility of bacteria by a number of methods mainly including disc diffusion, dilution (broth microdilution method, broth microdilution method and agar dilution), serial dilution (E-test) minimum inhibitory concentration (MIC) determination1,2, of which can be performed manually or automatically.
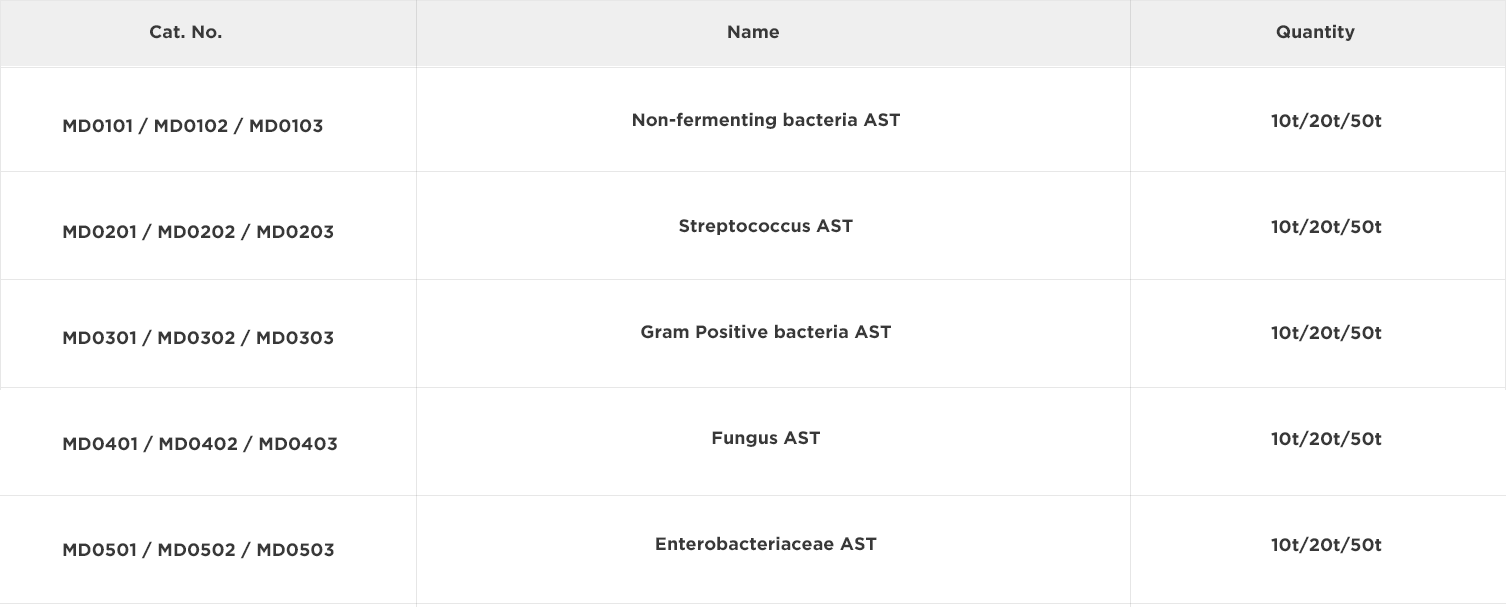
The MIC of an organism is defined as the minimum amount of antimicrobial agent required to inhibit the growth of the test organism over a specified time interval (which is related to the growth rate of the bacteria). The rapid and accurate determination of the MIC value for an antimicrobial agent/organism combination can significantly improve patient management and prognosis since it enables the prompt administration of appropriate antimicrobial agent therapy.
The Interpretive Categorical Results (SIR) should be interpreted by referring to the latest MIC breakpoint interpretation standard published by CLSI 3 or EUCAST 4
1.Miae Lee, Hae Sun Chung . Different antimicrobial susceptibility testing methods to detect ertapenem resistance in Enterobacteriaceae: VITEK2, MicroScan, Etest, disk diffusion, and broth microdilution, J Microbiol Methods, 2015, 112(197):87 91.
2. F.C. Tenover, Antibiotic Susceptibility Testing, Encyclopedia of Microbiology (Third Edition 2009: 67 77
3. Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing: approved standard. Document M100-S29, Clinical and Laboratory Standards Institute.
4. European Committee on Antimicrobial Susceptibility Testing. 2019. Breakpoint tables for interpretation of MICs and zone diameters, Version 9.0, European Committee on Antimicrobial Susceptibility Testing.
Address: NO.87 Jingbei Yi Rd, National Eco&Tech Zone, Zhengzhou, China
Email: info@autobio-diagnostics.com Tel: +86-371-6200-7036
Autobio Copyright Reserved for ICP 18006568. All Rights Reserved.



